The lymphatic system consists of a network of thin-walled vessels that drain fluid and particular matter from the interstitial spaces. However, unlike blood vessels, the lymphatics do not form a circular system. The unidirectional lymph flow recovers fluid from the periphery and returns it to the cardiovascular system (see Figure 1).
Lymphatic flow begins in the capillary networks (initial or terminal lymphatics). Lymphatic capillaries consist of a single layer of non-fenestrated endothelial cells sitting on an incomplete basement membrane. Prenodal collecting vessels drain the capillary networks and transport lymph to the regional lymph nodes. Lymphatic capillaries are not invested with mural cells, but collecting vessels do posses a smooth muscle cell layer (Schmid-Schonbein 1990b). Postnodal collecting vessels carry lymph between successive sets of lymph nodes or to larger lymphatic collecting vessels. Eventually, the larger lymph-collecting vessels drain lymph from the final set of lymph nodes into the lymph ducts. Lymph from the intestinal, hepatic and lumbar region drains into the cisterna chyli. The cisterna chyli acts as a collecting reservoir at the posterior end of the thoracic duct. The thoracic duct ascends upwards from the cisterna chyli. On its way it receives lymph from the rest of the body except from the upper right quadrant. The thoracic duct empties directly into the venous blood at the junction of the left internal jugular vein and the left subclavian vein. The lymph of the upper right quadrant is collected into the right lymphatic duct and returned into the veins at the right jugulo-subclavian confluence (for a general review of the anatomy and the function of the lymphatic system see Foster 1996; Swartz 2001).
Figure 1. Schematic view of the lymphatic system (adapted from Klein 1990)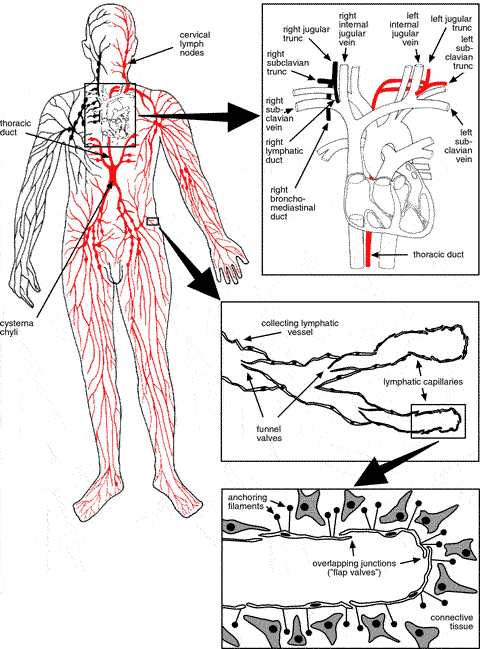
The heart is a powerful pump, and when blood enters the capillary bed it is still under a pressure of about 4-5 kPa. Due to this pressure 20 to 30 liters of plasma leak each day from the capillaries and become interstitial fluid (Landis and Pappenheimer 1963). The orthodox view is that about 90% of this extravasated fluid is reabsorbed at the venous end of the capillaries and post-capillary venules, driven by osmotic forces (Starling 1895-96). The remaining 10% are drained by the lymphatic vessels and returned to the cardiovascular system. For this reason lymphatic capillary beds are found in most vascular tissues. It should be noted, however, that not all experimental data supports the reabsorption theory (Bates et al. 1994). Two to five liters of thoracic duct lymph are formed in humans each day (Bierman et al. 1953; Linder and Blomstrand 1958). Lymph contains most of the components of plasma, although the concentration of high molecular weight components is lower due to the capillary filtration effect. The total protein concentration is about two thirds of that of serum (Bergstrom and Werner 1966; Werner 1966).
In addition to the drainage function, the lymphatics play multiple important roles in immune defence mechanisms. If body tissues are invaded by pathogens some are taken up together with the interstitial fluid by the lymphatics. The lymph passes through one or several lymph nodes before it enters the venous system. In collaboration with the antigen-presenting cells of the lymph nodes, T and B cells recognize non-self epitopes and mount an immune response. The activated immune cells proliferate in the lymph nodes and produce antibodies. Both cells and antibodies are delivered into the general circulation via the lymphatics. Not only the interstitial spaces but also the blood is screened via the lymph nodes, since about 50% of the plasma protein passes through the lymphatics each day (Klein 1990).
Another significant function of the lymphatics is the intestinal absorption and transport of long chain dietary triglycerides and lipophilic compounds such as fat-soluble vitamins or chlorinated organic compounds. After absorption from the gastrointestinal tract, blood and lymph capillaries compete for the transport of molecules to the systemic circulation. The majority of enterally administered compounds is absorbed into the portal blood since its throughput is about 500 times higher than that of the lymph. However, high molecular weight molecules and colloids are preferentially taken up by the lymphatics because of the highly permeable structure of the intestinal villous lymphatics (chylous vessels or lacteals). E.g. chylomicrons have a diameter of 200 to 800 nm and after being assembled and released by the enterocyte, gain access almost exclusively to chylous vessels. When pharmacological substances are absorbed by intestinal blood capillaries and transported via the portal blood to the liver, a large proportion can become inactivated (first-pass metabolic effect). Intestinal lymphatic absorption bypasses first-pass metabolic effects (Porter 1997).
The endothelial cells of the initial lymphatics lack tight junctions. Instead they are equipped with overlapping endothelial junctions, which function as mechanical flap valves. Filaments anchor the lymphatic endothelial cells into the surrounding connective tissue and are involved in the operation of the valves. Increased interstitial pressure forces these inter-endothelial valves to open and interstitial fluid and particulates gain access to the lymphatic lumen (Casley-Smith 1980).
In addition to fluid uptake via the valves, fluid transport by transcytosis and transendothelial channels plays a role (Leak 1971). Also both the hydrostatic and osmotic pressure gradient between lymphatic lumen and the interstitium have been suggested to contribute to lymph formation, although these forces cannot account for the removal of large proteins and particulate matter (Casley-Smith 1982a). The relative contributions of these mechanisms varies, and the mechanical valves seem to be especially important in situations of increased functional demand.
Lymphatic capillaries have no intrinsic contractility and depend entirely on extrinsic forces for lymph propulsion. The notable exception are the initial lymphatics in the bat wing, which do have their own contractile smooth muscle (Hogan and Unthank 1986). Alternating compression and dilation of the lymphatics by respiratory movement, contraction of skeletal and intestinal muscles, and the pressure pulse generated by adjacent arteries and arterioles propels lymph forward (Schmid-Schonbein 1990a). The directional flow of the lymph is maintained by funnel-shaped valves. The unit between two valves in the collecting lymphatics is termed lymphangion.
Contrary to the initial lymphatics, collecting lymphatics are contractile. Lymph flow from one lymphangion to the next is maintained against a pressure gradient and both extrinsic forces and contractions of the smooth muscle cell layer work together to pump the lymph against this gradient (Kinmonth and Taylor 1956; Olszewski and Engeset 1980). Median pressure in the initial lymphatics is close to atmospheric or interstitial values (Zweifach and Prather 1975). The pressure rises in the collecting lymphatics, and in the thoracic duct diastolic pressure ranges between -0.7 and 2.3 kPa and systolic pressure between 0.3 to 3 kPa (Kinnaert 1973).
Interstitial transport happens both by diffusion and convection. Movement of large molecules and particles in the interstitium is not always uniform (Jain and Gerlowski 1986), suggesting the existence of preferred pathways ("pre-lymphatic tissue channels"; Casley-Smith 1980), but the significance of these channels and even their existence has been questioned (Casley-Smith 1982b). Drainage can be achieved via "pre-lymphatic channels" as seen in the central nervous system: there is little doubt about the quasi-lymphatic function of the "pre-lymphatic" perivascular spaces in the brain. These spaces (Virchow-Robin spaces) connect to the cervical lymph nodes (Casley-Smith et al. 1976), and this connection is important for both drainage and immune response to brain infections (reviewed by Esiri and Gay 1990; Weller et al. 1996). Based on these functional criteria these channels have been occasionally classified as lymphatic, although they are devoid of an endothelial lining. However, capillary filtration is greatly reduced in the brain due to the blood brain barrier, and the majority of drainage is accomplished via the cerebrospinal fluid by the arachnoid villi and the adjacent specialized venous sinuses of the dura (Weed 1922/1923). There are several other vascular structures whose classification based on immunohistochemical and functional criteria remains inconclusive. These structures include the canal of Schlemm in the eye (Foets et al. 1992) and the perivascular spaces of the liver. It has been shown that genes specific for lymphatic endothelial cells, e.g. the receptor tyrosine kinase VEGFR-3, can be upregulated in non-lymphatic endothelial cells that fulfill a lymphatic function (Partanen et al. 2000). In the liver both blood vascular endothelial cells and hepatocytes line the perivascular spaces of the discontinuous liver capillary endothelium (Spaces of Disse). Despite little evidence they are assumed to fulfill a draining function, especially since the liver lobules themselves do not contain lymphatics (Niiro and O'Morchoe 1986; Trutmann and Sasse 1994). Also non-endothelial cells with an endothelial function have been shown to express VEGFR-3, e.g. the trophoblast cells of the placenta (Dunk and Ahmed 2001).
The matrix-lined channels seen in some melanomas are reminiscent of pre-lymphatic tissue channels. It was suggested that these channels participate in the tumor circulation, and such a behavior has been termed vasculogenic mimicry (Maniotis et al. 1999; Folberg et al. 2000). Tumor cells are known to contribute to the intima of tumor vessels, but the absence of endothelial cells in vasculogenic mimicry as described by Maniotis et al. remains controversial (McDonald et al. 2000).